If you’ve ever driven on icy roads, you’ll know just how treacherous they can be. How do road crews make the roads safer for travel? You may have seen them spraying a solution on the roads prior to a big storm, but what exactly is in that mixture and how does it help? With the extremely cold weather conditions across the country recently, this would be a timely topic for your students to explore.
I grew up in New Hampshire, and I can remember wondering why crews spread salt and sand on the roads. As it turns out, the salt lowered the freezing point of water which kept it from freezing at 32 degrees, and the sand made the roads less slippery providing traction for the vehicles.
Hands on Science Investigation and Research Ideas
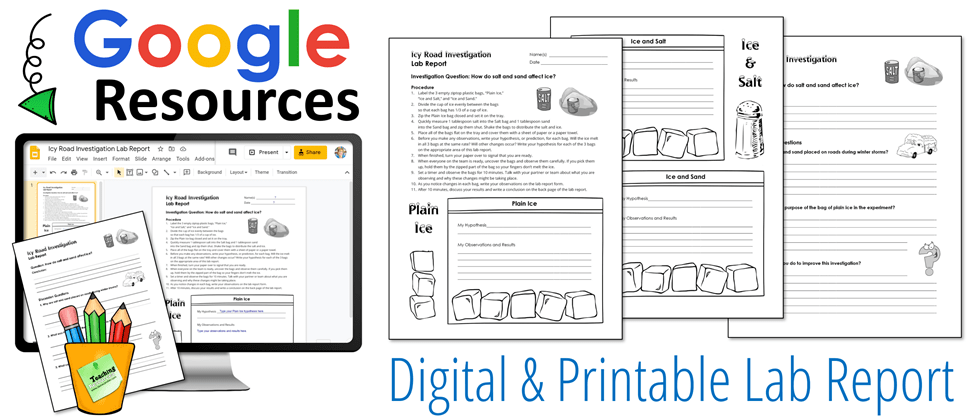
The Icy Road Investigation is a terrific way for students to explore these concepts. This hands-on science experiment involves placing ice cubes in zippered plastic bags with sand and salt so students can observe the effects of those two substances on the melting ice. The activity uses simple materials that you can easily find around the home, so it can be conducted in the classroom or at home for remote learning. This lesson includes teacher directions, student directions, and a lab report for students to complete as they conduct the investigation. Both printable and Google Slides versions of the lab report are included with the activity.
Where to Find More January Activities
Icy Road Investigation can be purchased alone or as a part of my January Activities pack. If you’re an upper elementary teacher who teaches many subjects in addition to science, you’ll find the January Activities pack to be a great value. In addition to the Icy Roads Investigation activity, this 28-page resource has over a dozen engaging lessons for January or winter including literacy, math, and social studies activities.
Preview the January Activities Pack on TpT
Icy Road Investigation Follow-up Activities
Researching and Investigating Other Ways to Make Icy Roads Safer
Recently scientists have been exploring other options that are not only environmentally safe – they actually save money. A few days ago I read an article with information about various solutions that seem to work even better than salt water. Would you be believe some states are using substances like cheese brine and a solution made from beets?
I wonder what other solutions might work to make roads safer in winter? A fun follow up to the Icy Roads Investigation would be to repeat the activity using a different solution to see how the new substance affects the results.
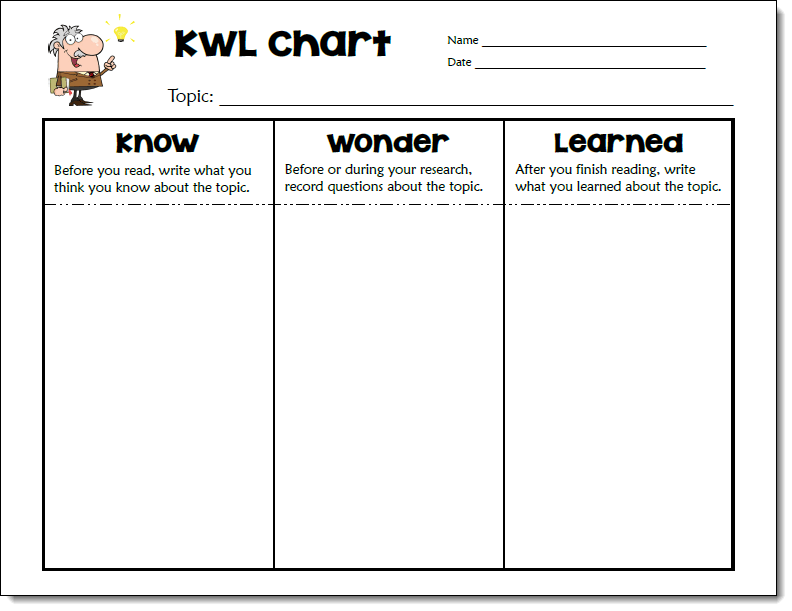
Know-Wonder-Learned Chart Freebie
You could also use a KWL chart to guide students through the process of researching how to make icy roads safer. Give each student a copy of the Know Wonder Learned chart below and display a copy for the class.
Write “Making Icy Roads Safer” on the topic line at the top of the chart. Then work with your students to complete the Know column by listing what they already know about the topic from the investigation and other sources. Next, ask your students to brainstorm questions they have about how roads can be made safer and list them in the Wonder column. Provide time for them to research the topic, take notes, and complete the Learned column. Finally, wrap up the lesson by having students share what they learned with the class.
Critique a Winter Weather News Report
Another engaging follow up activity is to have your students watch a video clip of a news reporter explaining why salt and sand are spread on highways and to discuss whether or not the information is scientifically correct. This idea was inspired by Debra, a 6th grade teacher who used my Icy Roads Investigation with her students and reviewed it on TpT.
The news report is titled Good Question: Why do Plows Sometimes Drop Sand not Salt? and the video clip shows a reporter from KUTV2News in Utah explaining why salt and sand are dropped on roads during a winter storm. However, the explanation for why salt is used is not scientifically correct. The reporter says that the purpose of the salt is to warm up the road, but if your students have completed the investigation, they will know that salt doesn’t actually make the road warmer. Salt simply lowers the freezing point of water to prevent ice from forming on roads at 32 degrees. Will your students catch this error? A written transcript of the news report is provided under the video which is helpful for analyzing what the reporter said and discussing why his explanation is not accurate.
Even if you don’t live where winter weather is a problem, your students have seen enough movies to know that snow and ice on the roads create dangerous driving conditions. Exploring how roads can be made safer is a timely science topic, and your student are sure to enjoy this hands-on activity and research project.